Abstract
The efficacy of COVID-19 vaccines in cancer populations remain unknown. Myeloproliferative neoplasms (MPNs), including chronic myeloid leukemia (CML), essential thrombocythemia (ET), polycythemia vera (PV), and myelofibrosis (MF) remain a vulnerable patient population and are immunocompromised due to impaired innate and adaptive immunity, heightened inflammation, and effects of ongoing treatment. We evaluate antibody and T-cell responses in MPN patients following completion of the BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna) COVID-19 vaccine series.
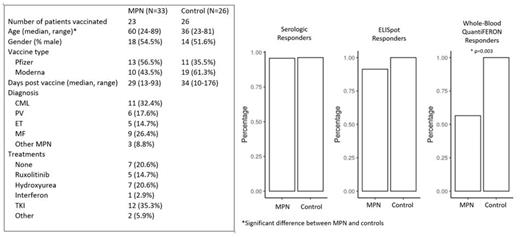
Patients with a known diagnosis of MPN presenting at Massachusetts General Hospital and eligible for COVID-19 vaccination were recruited. All participants gave informed consent and the study protocol was approved by the Institutional Review Board. 33 MPN patients were enrolled and 23 patients completed vaccination. Baseline and post-vaccination peripheral blood samples were collected and peripheral blood mononuclear cells (PBMCs) isolated. 26 vaccinated participants with no history of malignancy were included as healthy controls (PMID 33972942). Baseline characteristics are tabled below.
Qualitative ELISA for human IgG/A/M against SARS-CoV-2 spike protein using donor serum was performed per manufacturer instructions. Seroconversion occurred in 22/23 (96%) of MPN patients and 25/26 (96%) of healthy controls (Figure).
To measure SARS-CoV-2 T-cell immunity, an IFNγ ELISpot assay previously developed in convalescent and vaccinated healthy individuals was used. Freshly isolated PBMCs from patients were stimulated with commercially available overlapping 15mer peptide pools spanning the SARS-CoV-2 spike and nucleocapsid proteins. Given its size, the spike protein was split into two pools (Spike A or B). IFNγ-producing T-cells were quantified by counting the median spot forming units (SFU) per 2.5x10 5 PBMCs from duplicate wells. A positive threshold was defined as >6 SFUs per 2.5x10 5 PBMCs to either Spike A or B after subtraction of background, based on prior receiver operator curve (ROC) analysis of ELISpot responses (sensitivity 90% specificity 92%).
Post-vaccination ELISpot responses occurred in 21/23 (91%) of MPN patients and 26/26 (100%) of healthy controls (p=0.99) (Figure). The median SFU to total spike protein (Spike A+B) increased after vaccination in both MPN patients (0 to 38, p=0.02) and healthy controls (6 to 134, p=0.002). MPN patients had significantly lower median SFU's on post-vaccination ELISpot compared to healthy controls (38 vs 134, p=0.044), although this was not significant after adjusting for age in multivariable logistic regression. MF patients had the lowest seroconversion and ELISpot response rates, and lowest median post-vaccination SFUs, although this was not significant. There were no other differences in post-vaccination SFUs with regards to gender, vaccine type, number of days post-vaccine, treatment, and absolute lymphocyte count.
Whole-blood assay based on the in vitro diagnostic QuantiFERON TB Gold Plus assay was also used to assess T-cell response. Heparinized whole blood from donors was stimulated with S1 and S2 subdomains for the SARS-CoV-2 spike protein, with measurement of IFNγ released into plasma with the QuantiFERON ELISA. IFNγ release of >0.3 IU/mL was considered a positive threshold, based on prior ROC analysis (sensitivity and specificity 100%). MPN patients had significantly lower IFNγ response rates compared to healthy controls (57% versus 100%, p=0.003) (Figure).
Our findings demonstrate robust antibody and T-cell responses to BNT162b2 and mRNA-1273 vaccination in MPN patients, with >90% serologic and ELISpot responder rates. We detected subtle differences in T-cell responses in MPN patients compared to healthy controls. MPN patients had lower median post-vaccination ELISpot SFUs and lower rates of T-cell responses on IFNγ-whole blood assay compared to healthy controls. As the whole blood assay uses whole protein antigen rather than peptide pools, differences from ELISpot testing may reflect deficiencies in antigen processing and presentation. It is unclear whether these subtle differences translate into less clinical protection from COVID-19, or to what extent our results are confounded by the older age of MPN patients. Further evaluation of B and T-cell responses to COVID-19 vaccination in a larger sample size of MPN patients is warranted.
Neuberg: Pharmacyclics: Research Funding; Madrigal Pharmaceuticals: Other: Stock ownership. Maus: Atara: Consultancy; Bayer: Consultancy; BMS: Consultancy; Cabaletta Bio (SAB): Consultancy; CRISPR therapeutics: Consultancy; In8bio (SAB): Consultancy; Intellia: Consultancy; GSK: Consultancy; Kite Pharma: Consultancy, Research Funding; Micromedicine: Consultancy, Current holder of stock options in a privately-held company; Novartis: Consultancy; Tmunity: Consultancy; Torque: Consultancy, Current holder of stock options in a privately-held company; WindMIL: Consultancy; AstraZeneca: Consultancy; Agenus: Consultancy; Arcellx: Consultancy; Astellas: Consultancy; Adaptimmune: Consultancy; tcr2: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; century: Current equity holder in publicly-traded company; ichnos biosciences: Consultancy, Current holder of stock options in a privately-held company. Hobbs: AbbVie.: Consultancy; Incyte Corporation: Research Funding; Novartis: Consultancy; Bayer: Research Funding; Merck: Research Funding; Constellation Pharmaceuticals: Consultancy, Research Funding; Celgene/Bristol Myers Squibb: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal